SI andard Electrode Electrode Reaction Potentia V Au Au t 14
SI andard Electrode Electrode Reaction Potentia (V) Au Au t 1.421 HO 1.220 PI e P1 1 A A Increasingly inert (cathodic) o, 2H,o 4 (OH) +0.401 noble u 0.340 0.000 -0.126 -0.130 Sn 0.250 0.277 o o 403 d active 0.440 0.744 r Increasingly active (anodic) -0.7 Zn Al Al 2.363 Na 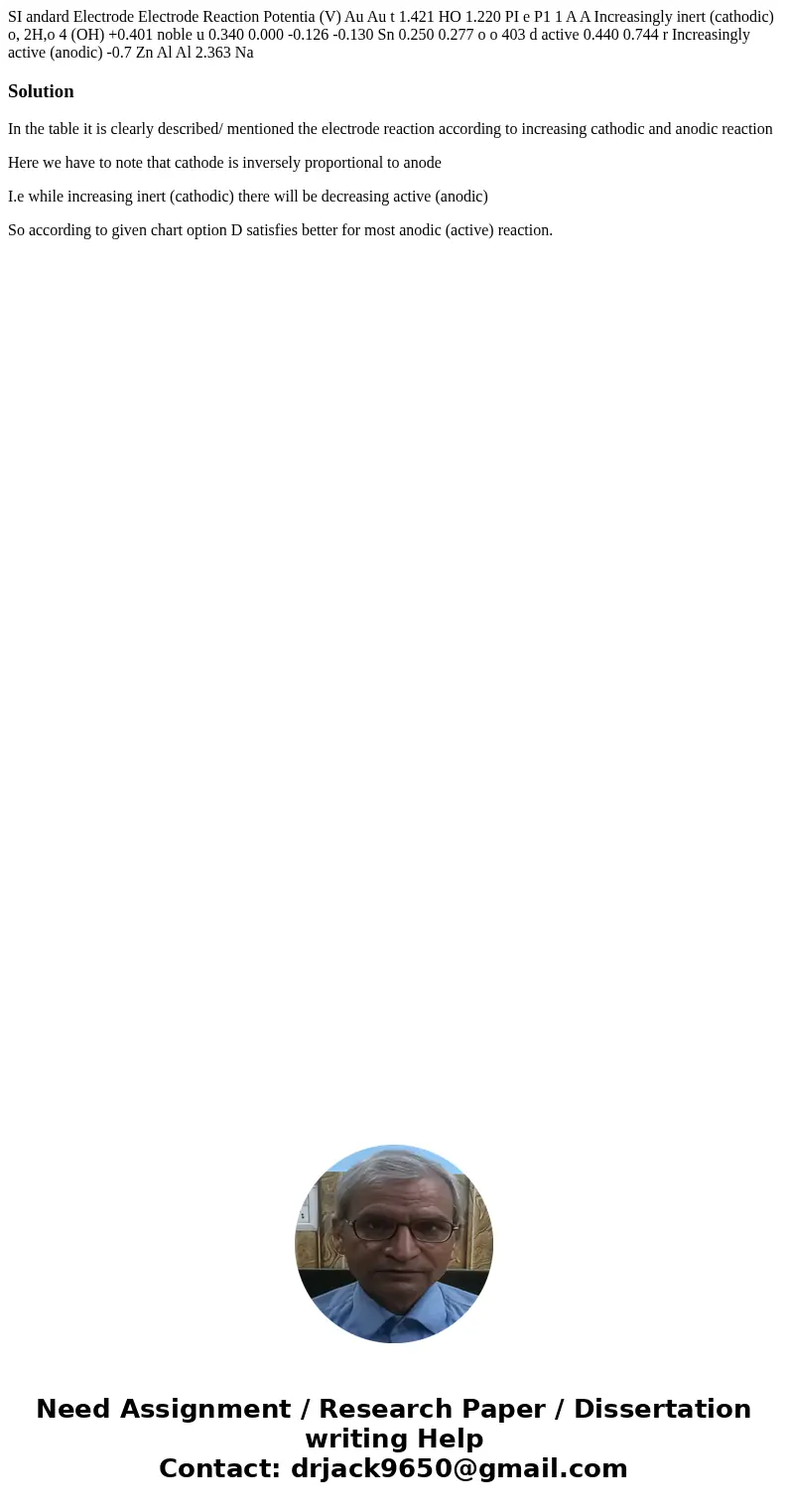
Solution
In the table it is clearly described/ mentioned the electrode reaction according to increasing cathodic and anodic reaction
Here we have to note that cathode is inversely proportional to anode
I.e while increasing inert (cathodic) there will be decreasing active (anodic)
So according to given chart option D satisfies better for most anodic (active) reaction.

 Homework Sourse
Homework Sourse