Derive a general expression for the concentration of tritium
Derive a general expression for the concentration of tritium in a lake as a function of time.
Solution
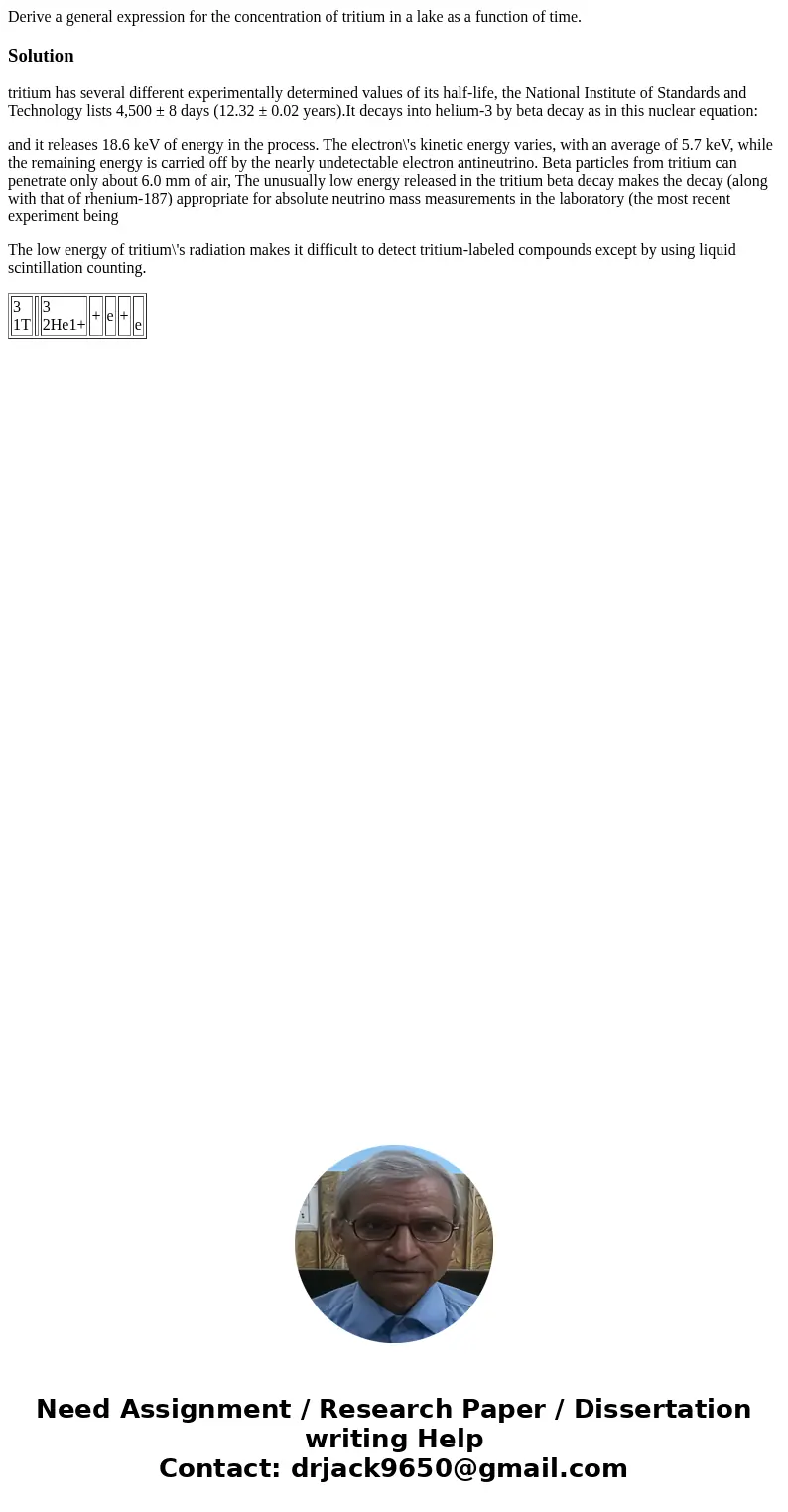
tritium has several different experimentally determined values of its half-life, the National Institute of Standards and Technology lists 4,500 ± 8 days (12.32 ± 0.02 years).It decays into helium-3 by beta decay as in this nuclear equation:
and it releases 18.6 keV of energy in the process. The electron\'s kinetic energy varies, with an average of 5.7 keV, while the remaining energy is carried off by the nearly undetectable electron antineutrino. Beta particles from tritium can penetrate only about 6.0 mm of air, The unusually low energy released in the tritium beta decay makes the decay (along with that of rhenium-187) appropriate for absolute neutrino mass measurements in the laboratory (the most recent experiment being
The low energy of tritium\'s radiation makes it difficult to detect tritium-labeled compounds except by using liquid scintillation counting.
| 3 1T | 3 2He1+ | + | e | + | e |
 Homework Sourse
Homework Sourse