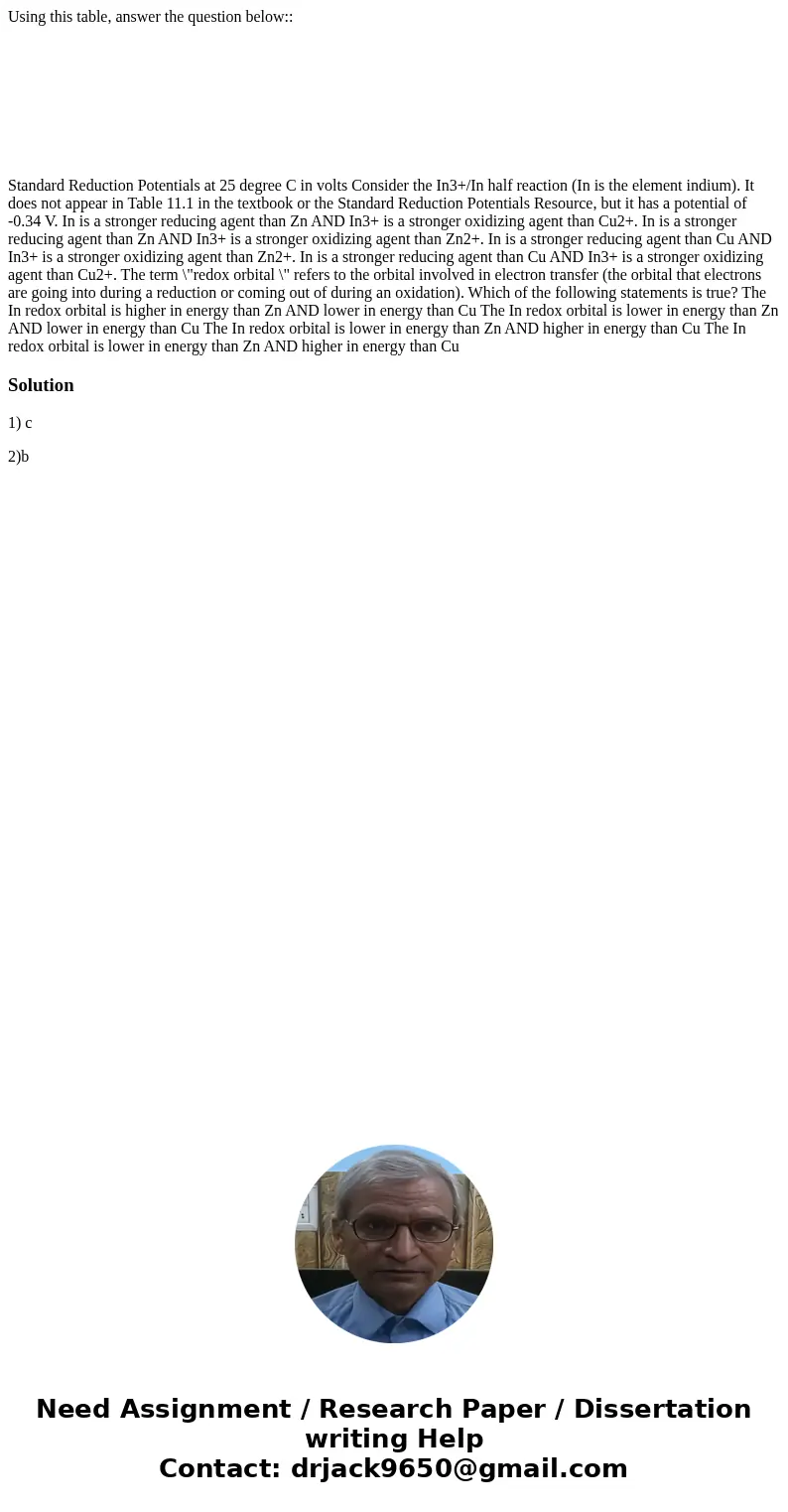
Using this table, answer the question below::
Standard Reduction Potentials at 25 degree C in volts Consider the In3+/In half reaction (In is the element indium). It does not appear in Table 11.1 in the textbook or the Standard Reduction Potentials Resource, but it has a potential of -0.34 V. In is a stronger reducing agent than Zn AND In3+ is a stronger oxidizing agent than Cu2+. In is a stronger reducing agent than Zn AND In3+ is a stronger oxidizing agent than Zn2+. In is a stronger reducing agent than Cu AND In3+ is a stronger oxidizing agent than Zn2+. In is a stronger reducing agent than Cu AND In3+ is a stronger oxidizing agent than Cu2+. The term \"redox orbital \" refers to the orbital involved in electron transfer (the orbital that electrons are going into during a reduction or coming out of during an oxidation). Which of the following statements is true? The In redox orbital is higher in energy than Zn AND lower in energy than Cu The In redox orbital is lower in energy than Zn AND lower in energy than Cu The In redox orbital is lower in energy than Zn AND higher in energy than Cu The In redox orbital is lower in energy than Zn AND higher in energy than Cu
1) c
2)b