flask No time mirn HT H201 1IFl ratemin mh 176 105 M mr 176
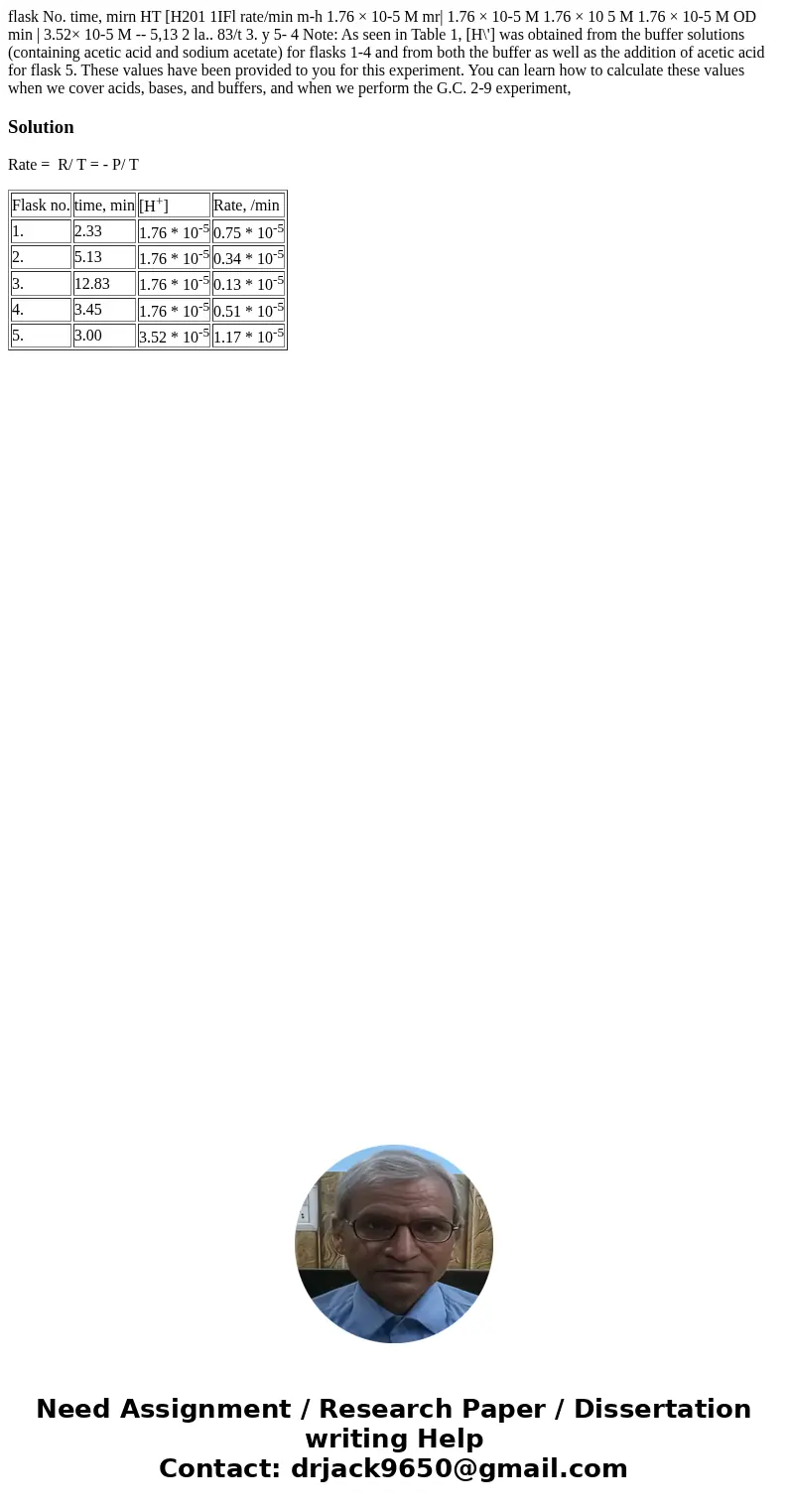
flask No. time, mirn HT [H201 1IFl rate/min m-h 1.76 × 10-5 M mr| 1.76 × 10-5 M 1.76 × 10 5 M 1.76 × 10-5 M OD min | 3.52× 10-5 M -- 5,13 2 la.. 83/t 3. y 5- 4 Note: As seen in Table 1, [H\'] was obtained from the buffer solutions (containing acetic acid and sodium acetate) for flasks 1-4 and from both the buffer as well as the addition of acetic acid for flask 5. These values have been provided to you for this experiment. You can learn how to calculate these values when we cover acids, bases, and buffers, and when we perform the G.C. 2-9 experiment,
Solution
Rate = R/ T = - P/ T
| Flask no. | time, min | [H+] | Rate, /min |
| 1. | 2.33 | 1.76 * 10-5 | 0.75 * 10-5 |
| 2. | 5.13 | 1.76 * 10-5 | 0.34 * 10-5 |
| 3. | 12.83 | 1.76 * 10-5 | 0.13 * 10-5 |
| 4. | 3.45 | 1.76 * 10-5 | 0.51 * 10-5 |
| 5. | 3.00 | 3.52 * 10-5 | 1.17 * 10-5 |

 Homework Sourse
Homework Sourse