When propanol 2CH3CH2CH2OH is combusted such as when in a g
When propanol (2CH3CH2CH2OH ) is combusted, such as when in a gasoline blend, the following reaction occurs:
2CH3CH2CH2OH+9O2>>>6CO2+8H2O
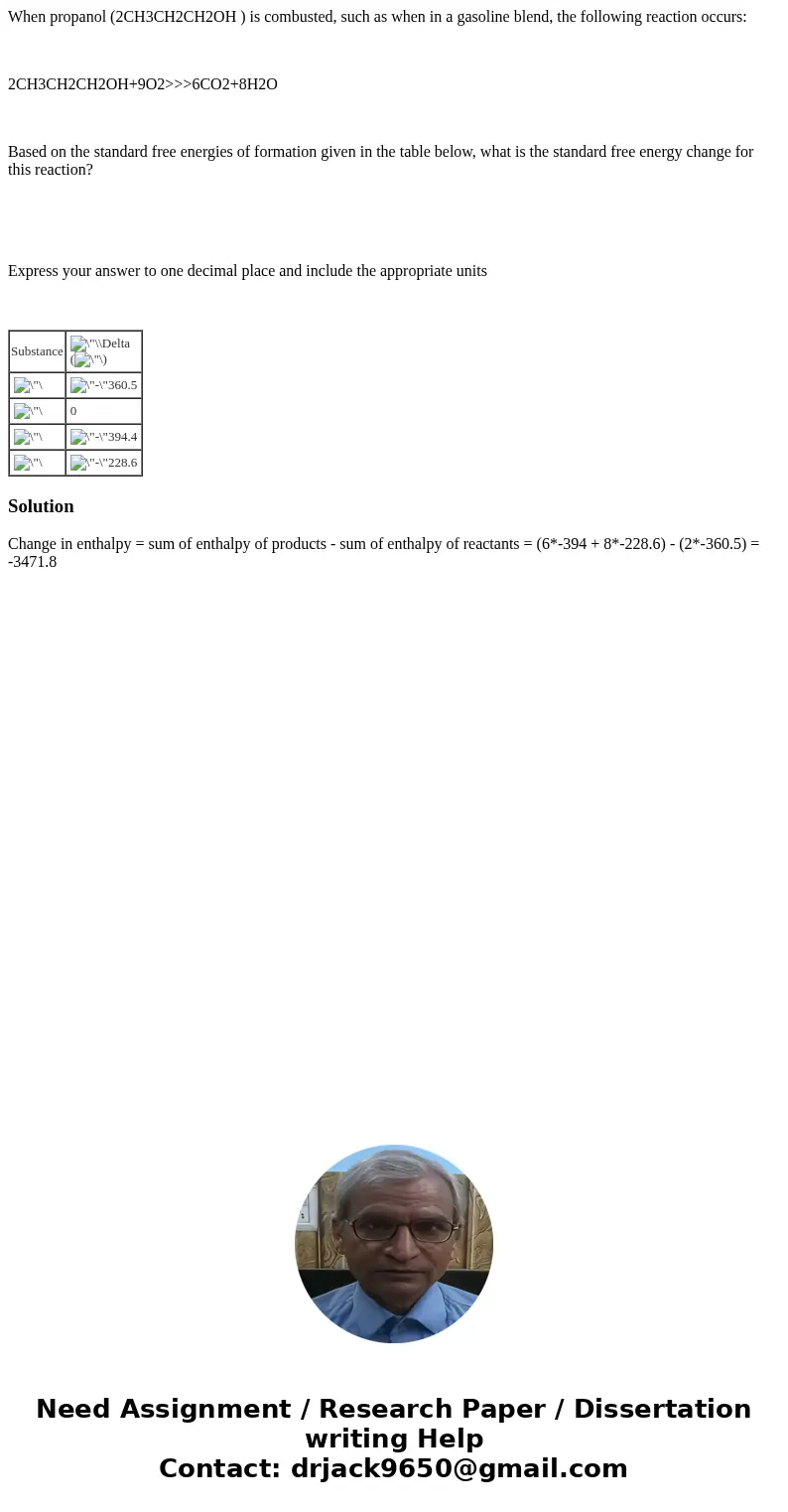
Based on the standard free energies of formation given in the table below, what is the standard free energy change for this reaction?
Express your answer to one decimal place and include the appropriate units
| Substance | ( |
| 0 | |
Solution
Change in enthalpy = sum of enthalpy of products - sum of enthalpy of reactants = (6*-394 + 8*-228.6) - (2*-360.5) = -3471.8
 Homework Sourse
Homework Sourse