Now make up 1L stock solutions of each of the following Well
Now, make up 1L stock solutions of each of the following (We’ll skip the vitamins):
2.94 g NaF
17.4 g SrCl2.6H2O
6.8 g KH2PO4
0.11 g FeCl3.6H2O
0.012 g ZnSO4.7H2O
0.04 g MnCl2.4H2O
0.027 g Na2MoO4.2H2O
0.06 g CoCl2.6H2O
0.025 g CuSO4.5H2O
Add 1 mL each of the first 5 stock solutions to the seawater mixture above. Add 100 mL of the Mn, Zn, and Mo stock solutions to the seawater mixture above. Add 10 mL of the Co and Cu stock solutions to the seawater mixture above. What are the final concentrations of each trace element in the seawater solution (F, Sr, P, Fe, Zn, Mn, Mo, Co, and Cu (in mmol/L)? What is the amount of each of these constituents in g/kg? Do these concentrations seem about right?(20 points)
Solution
Below are concentrations in 1 L stock solution.
2.94 g NaF
17.4 g SrCl2.6H2O
6.8 g KH2PO4
0.11 g FeCl3.6H2O
0.012 g ZnSO4.7H2O
0.04 g MnCl2.4H2O
0.027 g Na2MoO4.2H2O
0.06 g CoCl2.6H2O
0.025 g CuSO4.5H2O
After addition of 1 mL of first 5 stock solutions we have,
2.94 mg NaF
17.4 mg SrCl2.6H2O
6.8 mg KH2PO4
0.11 mg FeCl3.6H2O
0.012 mg ZnSO4.7H2O
After addition of 100 mL of Mn, Zn, Mo solutions we have,
2.94 mg NaF
17.4 mg SrCl2.6H2O
6.8 mg KH2PO4
0.11 mg FeCl3.6H2O
0.012 mg ZnSO4.7H2O
0.004 g MnCl2.4H2O
0.0012 g ZnSO4.7H2O, Total = 0.001212 g
0.0027 g Na2MoO4.2H2O
After addition of 10 mL of Co, Cu solutions we have,
2.94 mg NaF
17.4 mg SrCl2.6H2O
6.8 mg KH2PO4
0.11 mg FeCl3.6H2O
0.004 g MnCl2.4H2O
0.001212 g ZnSO4.7H2O
0.0027 g Na2MoO4.2H2O
0.0006 g CoCl2.6H2O
0.00025 g CuSO4.5H2O
Total volume = 5 + 300 + 20 = 365 mL
Total mass = 36.012 mg
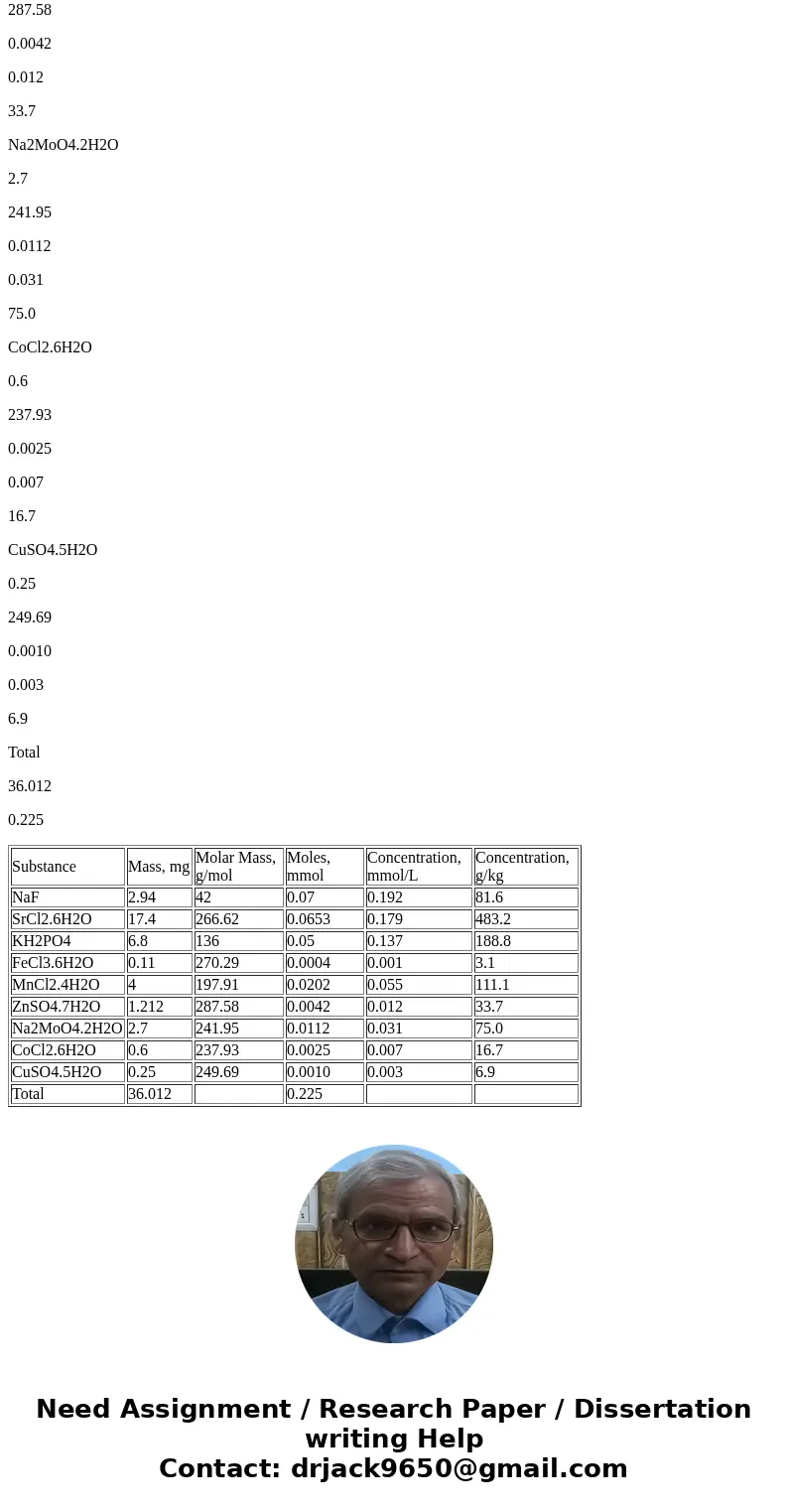
Below table gives concentrations of each substance:
Substance
Mass, mg
Molar Mass, g/mol
Moles, mmol
Concentration, mmol/L
Concentration, g/kg
NaF
2.94
42
0.07
0.192
81.6
SrCl2.6H2O
17.4
266.62
0.0653
0.179
483.2
KH2PO4
6.8
136
0.05
0.137
188.8
FeCl3.6H2O
0.11
270.29
0.0004
0.001
3.1
MnCl2.4H2O
4
197.91
0.0202
0.055
111.1
ZnSO4.7H2O
1.212
287.58
0.0042
0.012
33.7
Na2MoO4.2H2O
2.7
241.95
0.0112
0.031
75.0
CoCl2.6H2O
0.6
237.93
0.0025
0.007
16.7
CuSO4.5H2O
0.25
249.69
0.0010
0.003
6.9
Total
36.012
0.225
| Substance | Mass, mg | Molar Mass, g/mol | Moles, mmol | Concentration, mmol/L | Concentration, g/kg |
| NaF | 2.94 | 42 | 0.07 | 0.192 | 81.6 |
| SrCl2.6H2O | 17.4 | 266.62 | 0.0653 | 0.179 | 483.2 |
| KH2PO4 | 6.8 | 136 | 0.05 | 0.137 | 188.8 |
| FeCl3.6H2O | 0.11 | 270.29 | 0.0004 | 0.001 | 3.1 |
| MnCl2.4H2O | 4 | 197.91 | 0.0202 | 0.055 | 111.1 |
| ZnSO4.7H2O | 1.212 | 287.58 | 0.0042 | 0.012 | 33.7 |
| Na2MoO4.2H2O | 2.7 | 241.95 | 0.0112 | 0.031 | 75.0 |
| CoCl2.6H2O | 0.6 | 237.93 | 0.0025 | 0.007 | 16.7 |
| CuSO4.5H2O | 0.25 | 249.69 | 0.0010 | 0.003 | 6.9 |
| Total | 36.012 | 0.225 |



 Homework Sourse
Homework Sourse